20+ Protons Neutrons Electrons Calculator
Biology 20 calculators. Atoms of chlorine contain 17 protons.
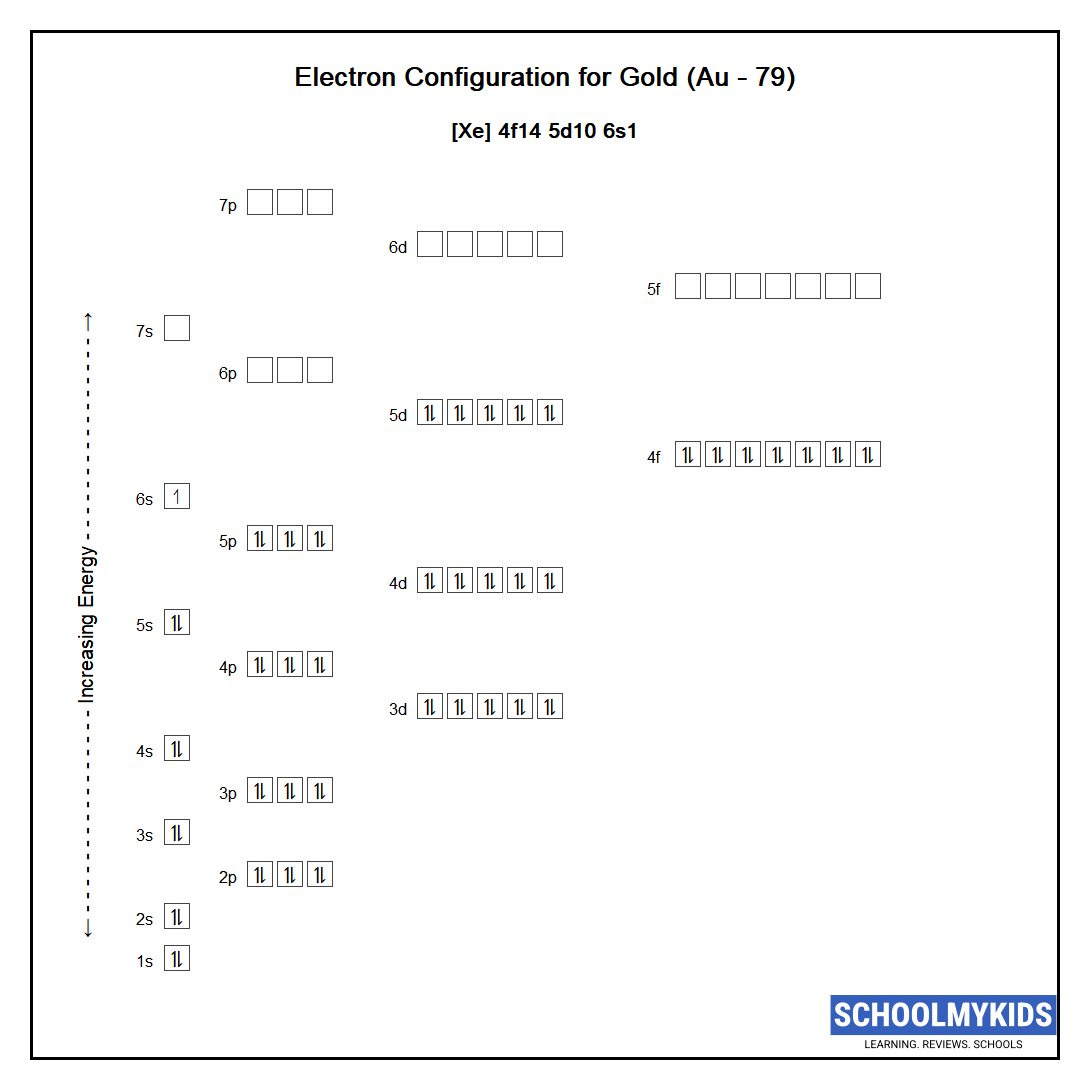
Au Gold Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
Co has 27 protons 27 electrons and 33 neutrons.

. How many protons neutrons and electrons are in atoms of these isotopes. It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common. Write the complete electron configuration for each isotope.
To use this online calculator for Number of Neutrons enter Mass Number A Atomic Number Z and hit the calculate button. B The nucleus of an atom is made up of protons and neutrons. I has 53 protons 53 electrons and 78 neutrons.
Every nuclide has a chemical element symbol E as well as an atomic number Z the number of protons in the nucleus and a mass number A the total number of protons and neutrons in the nucleus. Filled shells such as the filled shell of 50 protons for tin confers unusual stability on the nuclide. The symbol for the element is as shown below.
Which of the following correctly represents the electronic distribution in the Mg atom. In its amorphous form it is a brown powder. Ferrum and atomic number 26.
A_ZE An example is neon which has the element symbol Ne atomic number 10 and mass number 20. Relative isotopic mass Similar terms for different quantities Mass defects in atomic masses The ratio of atomic mass to mass. Those are much smallera protons diameter is about 17 femtometers across and a femtometer is a millionth of a nanometer.
Relative mass relative charge proton 1 1 neutron 1 0 electron very small 1 Figure 4 i Explain using the information in Figure 3 and Figure 4 why atoms of chlorine. 22 Bonding and Lattices. Browse our listings to find jobs in Germany for expats including jobs for English speakers or those in your native language.
ASCII characters only characters found on a standard US keyboard. Chapter 20 Electric Potential and Electrical Potential Energy Q102GP The electric potential a distance r from a point charge q is 270 104 V. Iron ˈ aɪ ə n is a chemical element with symbol Fe from Latin.
25 Formation of Minerals. One meter farther away from the charge the potential is 6140 V. The average potential varies.
Particles of the shell structure of nuclei are neutrons and protons. Find the charge q and the initial distance r. Chapter 21 Geological History of Western Canada.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5. Particles of the shell structure of an atom are electrons. This recommendable online calculator can find the atomic mass of any element by adding the number of neutrons and protons that is more beneficial for both students and tutors.
In its crystalline form it is a brittle dark lustrous metalloid. Must contain at least 4 different symbols. How to calculate Number of Neutrons using this online calculator.
Structure of the Atom Class 9 MCQs Questions with Answers. Stability of isotopes is affected by the ratio of protons to neutrons and also by presence of certain magic numbers of neutrons or protons which represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus.
Boron is a chemical element with the symbol B and atomic number 5. As well as that in the case of extremely high velocities comparable to the speed of light or extremely small masses comparable to the mass of atoms. You can refer to NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
As the lightest element of the boron group it has three valence electrons for forming covalent bonds resulting in many compounds such as boric acid the mineral sodium borate and the ultra-hard crystals of boron. From the source of Wikipedia. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7.
Number of Neutrons is denoted by n 0 symbol. And the mass of the body is much higher than the mass of atomic parts electrons protons and neutrons. 21 Electrons Protons Neutrons and Atoms.
6 to 30 characters long. Magic numbers Z 21018365486 and so on. Chapter 20 Electric Potential and Electrical Potential Energy Q103GP.
Magic numbers Z 2820285082126 and so on. Here is how the Number of Neutrons calculation can be explained with given input values - 20 37-17. Figure 4 shows some information about a proton a neutron and an electron.
The average potential of nuclei is different from that of an atom. The next step would be much hardermanipulation of the subatomic particles in an atoms nucleus like protons and neutrons.

How To Find The Number Of Protons Neutrons And Electrons

How To Find The Number Of Protons Electrons Neutrons For Silver Ag Youtube
Pbl Wheeler S Thoughts On Teaching

Total Number Stock Illustrations 1 006 Total Number Stock Illustrations Vectors Clipart Dreamstime

Infinite Dimensions Stock Illustrations 76 Infinite Dimensions Stock Illustrations Vectors Clipart Dreamstime

Atom Structure Concepts Atomic Energy Its Properties

Snc1p

Effects Of Charge State Charge Distribution And Structure On The Ion Mobility Of Protein Ions In Helium Gas Results From Trajectory Method Calculations The Journal Of Physical Chemistry A
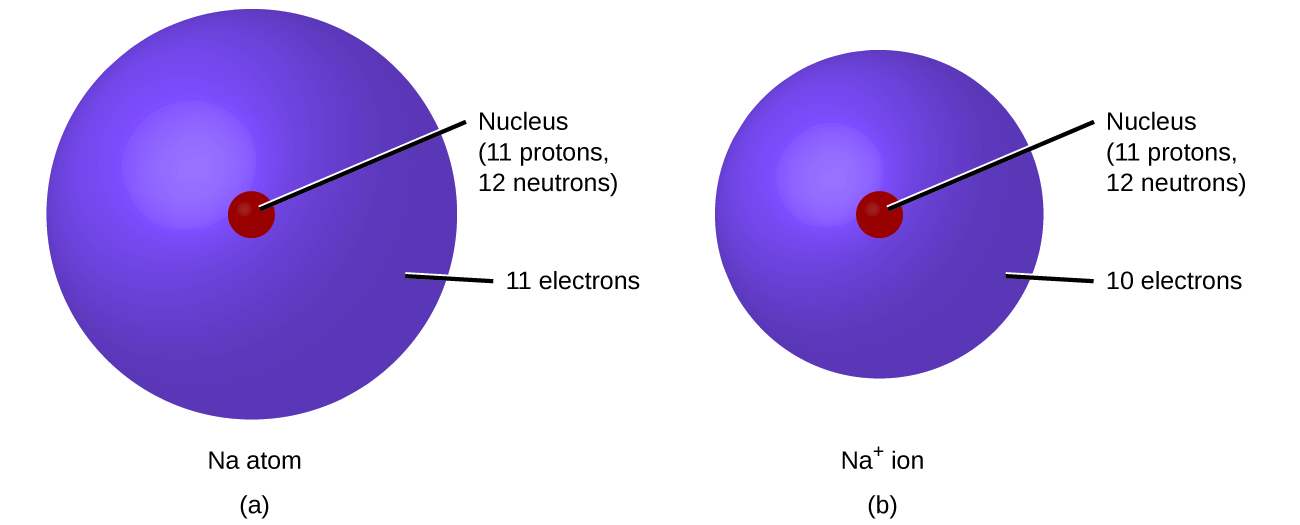
3 3 Predicting Charges Of Ions Chemistry Libretexts

Tea Pb Manual Pdf Lecture Teaching Method

Tweets With Replies By Rockstarrucker Rockstarrucker Twitter
Infinite Dimensions Stock Illustrations 76 Infinite Dimensions Stock Illustrations Vectors Clipart Dreamstime

Atomic Mass Calculator Calculate Neutrons Protons Electrons

Amber Harrison Amber13harrison Twitter
How Many Times Is The Weight Of Proton Greater Than Mass Of Electron Quora

Atomic Mass Calculator Calculate Neutrons Protons Electrons

Atomic Mass Calculator Calculate Neutrons Protons Electrons